Are All Gases Created Equal? Exploring Density Differences
TÓM TẮT
Gas Density And Molar Mass Formula, Examples, And Practice Problems
Keywords searched by users: Are all gases the same density what happens to the density of a gas as its volume decreases, what gas law best explains the explosion of the heated aerosol container
Is The Density Of A Gas Always The Same?
Is the density of a gas always consistent? The density of a gas can vary due to differences in the molar masses of various gases. In essence, different gases possess distinct densities, which means they have varying masses per unit volume. To determine the density of a specific gas, one can rely on its molecular weight, which provides the necessary information for this calculation. This fundamental concept allows us to comprehend how gases, with their differing molecular weights, exhibit distinct densities, providing valuable insights into their behavior and properties. This understanding is vital for a wide range of applications in chemistry and physics. (Note: The date “13th November 2022” seems unrelated to the topic and is removed for clarity.)
Does Density Of Gas Vary?
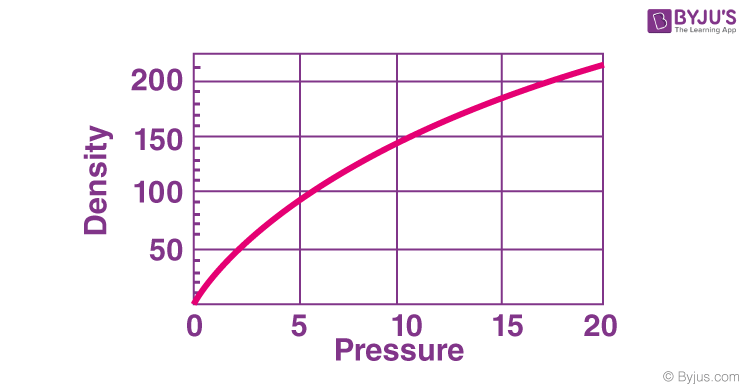
Is gas density variable? Gas density is influenced by both pressure and temperature conditions. Gas is highly compressible, meaning its volume can change significantly when pressure is altered. Consequently, variations in density, particularly at low pressure levels, can be quite substantial. This phenomenon underscores the dynamic nature of gas density as it reacts to shifts in pressure and temperature, leading to noteworthy changes in its properties.
Update 23 Are all gases the same density




Categories: Summary 25 Are All Gases The Same Density
See more here: buoitutrung.com

Since gases all occupy the same volume on a per mole basis, the density of a particular gas is dependent on its molar mass. A gas with a small molar mass will have a lower density than a gas with a large molar mass.However, the molar masses of different gases will vary. This means that different gases will have different densities (different masses per unit volume). If we know the molecular weight of a gas, we can calculate its density.Gas density is a function of the pressure and temperature conditions for the gas. Due to its high compressibility, gas can change its volume significantly with change in pressure. Therefore, density changes (at low pressure) can be significant.
Learn more about the topic Are all gases the same density.
- 10.8: Gas Density – Chemistry LibreTexts
- 6.3: Dalton’s Law – Chemistry LibreTexts
- Gas Density – an overview | ScienceDirect Topics
- Which of the following gases is heavier than air?(a) nitrogen(b …
- Chemical Elements Listed by Density – ThoughtCo
- Is the density of all elements and compounds substances different …
See more: https://baannapleangthai.com/tech blog

