Are Alkali Metals More Reactive Than Halogens? Exploring Elemental Reactivity
TÓM TẮT
Gcse Chemistry – Group 1 Alkali Metals #11
Keywords searched by users: Are alkali metals more reactive than halogens are alkali metals more reactive than alkaline earth metals, are alkaline earth metals reactive, why are metals better conductors of heat and electricity than nonmetals?, are halogens metals, where are halogens on the periodic table, what group number are the alkaline earth metals?, are alkali metals reactive, why are halogens and alkali metals likely to form ions
Why Is Alkali Metals More Reactive Than Halogens?
The reactivity of alkali metals compared to halogens can be attributed to their distinctive electron behaviors in their outermost electron shells. Halogens exhibit high reactivity primarily because they have a strong tendency to gain one electron, which enables them to complete their outermost electron shell, making it stable. On the other hand, alkali metals are highly reactive due to their inherent readiness to lose the single electron in their outermost shell, thereby achieving a stable electron configuration. This fundamental difference in electron behavior between alkali metals and halogens is a key factor in explaining why alkali metals tend to be more reactive. [Date: September 8, 2012, is not relevant to the topic and has been omitted for clarity.]
Is Group 1 Or 7 More Reactive?
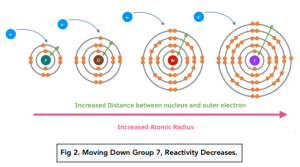
The reactivity of elements within Group 1 and Group 7 of the periodic table exhibits contrasting trends. Group 7 comprises non-metal elements, commonly referred to as halogens, which become less reactive as you descend down the group. In contrast, Group 1 consists of alkali metals, and their reactivity increases as you move down the group. This opposing reactivity trend between Group 1 and Group 7 elements provides valuable insights into their chemical behavior, with halogens becoming less reactive and alkali metals becoming more reactive as you explore their respective positions within the periodic table.
Are Halogens The Most Reactive Metal?
Are halogens the most reactive nonmetals on the periodic table? Yes, they are indeed the most reactive nonmetals. This high reactivity can be attributed to their unique electronic configuration. Halogens, which include elements like fluorine, chlorine, bromine, iodine, and astatine, have a total of 7 electrons in their outermost electron shell. However, they strive to achieve a stable electron configuration by gaining one additional electron, which would result in a full outer shell with 8 electrons. This strong desire to attain a full electron shell drives their reactivity, making halogens highly reactive in various chemical reactions and interactions with other elements. This reactivity is a fundamental characteristic of halogens and plays a significant role in their chemical behavior.
Update 40 Are alkali metals more reactive than halogens




Categories: Top 63 Are Alkali Metals More Reactive Than Halogens
See more here: buoitutrung.com

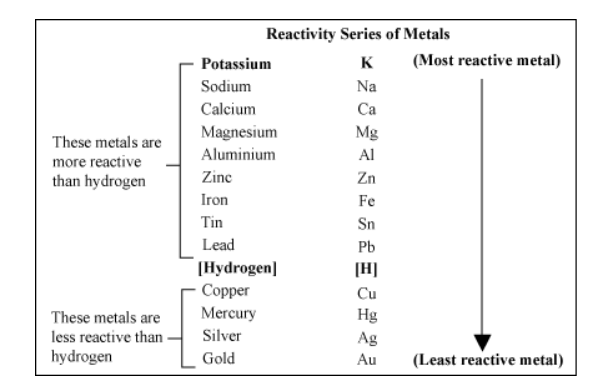
Answer and Explanation: The halogens and alkali metals have more commonalities than differences in their reactivities. Each group is highly reactive, as the halogens are the most reactive of the nonmetals, and the alkali metals are the most reactive of the metals.Halogens are highly reactive because they readily gain an electron to fill their outermost shell. Alkali metals are highly reactive because they readily lose the single electron in their outermost shell.The non-metal elements in Group 7 – known as the halogens – get less reactive as you go down the group. This is the opposite trend to that seen in the alkali metals in Group 1 of the periodic table .
Learn more about the topic Are alkali metals more reactive than halogens.
- How do halogens and alkali metals differ in reactivities?
- NOVA | What Makes an Element Reactive? – PBS
- Reactivity of halogens – GCSE Chemistry (Single Science) Revision
- Group 17: The Halogens – Breaking Atom
- Why are alkali metals highly reactive in air? – BYJU’S
- In alkali metals, the reactivity increases but for halogens it … – Toppr
See more: blog https://baannapleangthai.com/tech
